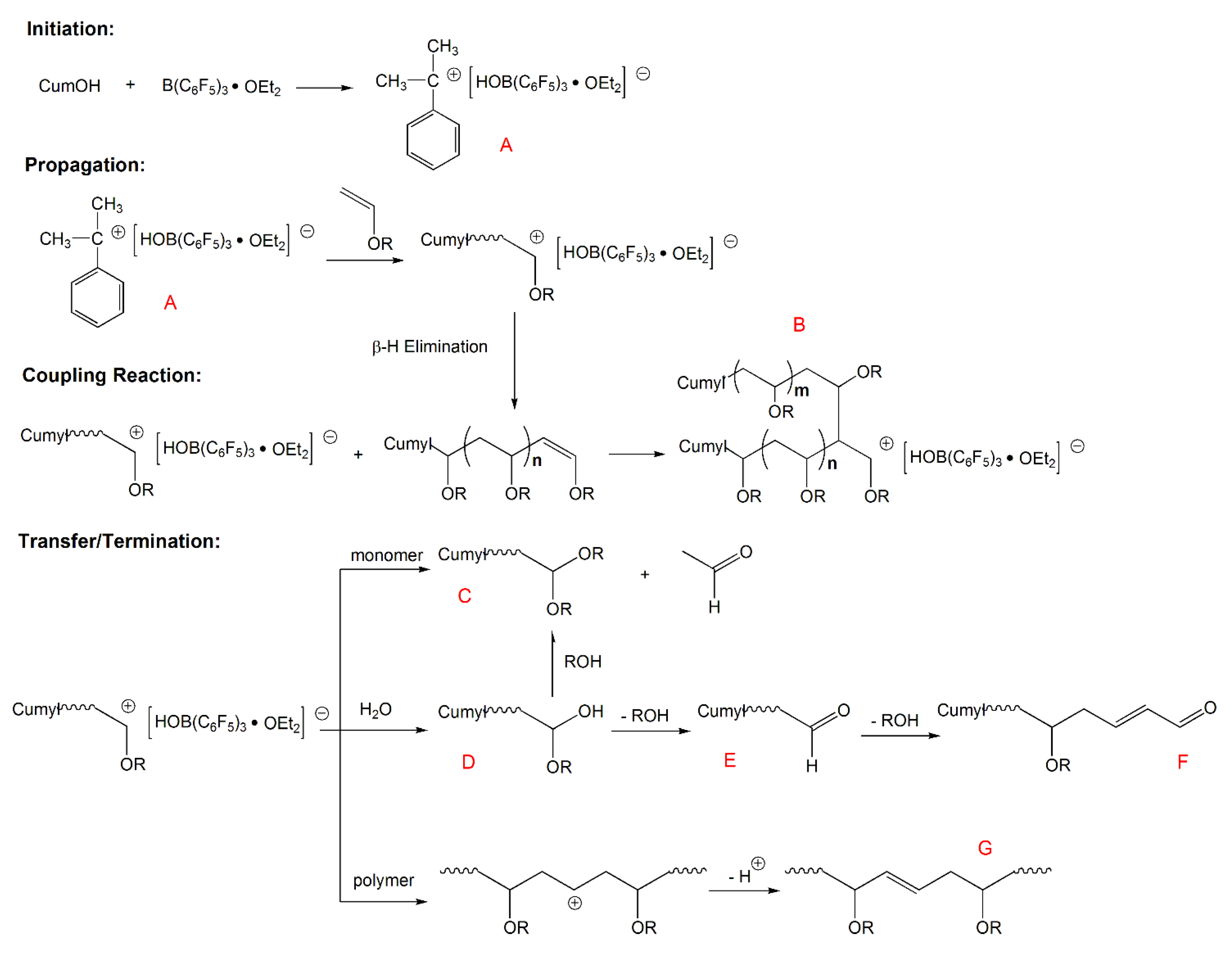
Last checked at 12 48am submit quiz fee 80 3 5 k 4 6 7 8.
Methyl vinyl ether resonance structures.
Structure 1 1967 68 239 248 spectra and structure of methyl acrylate and vinyl acetate 241 carbonyl stretching frequencies of vinyl esters by proposing that the resonance in vinyl acetate occurred through only the vinyl group and ether oxygen with the carbonyl group being excluded.
According to a classification scheme 7 an estimated bcf of 3 src from an estimated log kow of 0 42 2 and a regression.
Methyl vinyl ether has the lewis structure shown below.
Cid 87076889 c6h12o2 cid 87076889 structure chemical names physical and chemical properties classification patents literature biological activities.
In the provided space show the 3 d hybrid orbital bonding picture of methyl vinyl ether.
Methyl vinyl ether 107 25 5.
The general structure is r 2 c cr or where r h alkyl or aryl a common subfamily of enol ethers are vinyl ethers with the formula roch ch 2 important enol ethers include the reagent 3 4 dihydropyran and the monomers methyl vinyl ether and ethyl vinyl ether.
C indicate the location of lone pairs.
Methyl vinyl ether is an organic compound with the chemical formula ch 3 och ch 2 a colorless gas it is the simplest enol ether it is used as a synthetic building block as is the related compound ethyl vinyl ether a liquid at room temperature.
H c draw your resonance on paper and upload your answer upload choose a file previous next no new data to save.
Indeed resonance structure c1 makes a greater contribution to the hybrid than structure c1.
In resonance structure c1 every atom has a filled valence shell.
Structure properties spectra suppliers and links for.
Thus the contributions of the resonance form are greatest in methyl vinyl ether and least in t butyl vinyl ether.
Vinyl methyl ether is expected to undergo hydrolysis in the environment based on hydrolysis half lives of 9 5 hours 40 days and 10 9 years at ph 5 6 and 9 respectively in structurally similar butyl vinyl ether 6.
10 question 2 draw resonance structure s of vinyl methyl ether structure show below.
In organic chemistry an enol ether is an alkene with an alkoxy substituent.
On the other hand the resonance in alkyl vinyl ether must lead to a reduction in the olefinic character of the vinyl group and consequently the terminal methyl ene protons will become more equivalent.